The landscape of biopharmaceutical manufacturing has undergone seismic shifts in recent years, with cell culture processes standing at the very heart of production scalability and economic viability. As therapeutic modalities grow increasingly complex and market pressures intensify, the pursuit of cost reduction in cell culture—spanning both media formulation and bioreactor operations—has evolved from a secondary concern to a primary strategic imperative. This is not merely about trimming expenses but about fundamentally reengineering processes to achieve greater accessibility, sustainability, and efficiency without compromising product quality or regulatory compliance.
Cell culture media, once considered a somewhat standardized input, is now recognized as one of the most significant and adjustable cost drivers in upstream bioprocessing. The formulation itself—the precise cocktail of amino acids, vitamins, salts, growth factors, and other supplements—directly influences cell growth, productivity, and the consistency of the final product. Historically, many processes relied on serum-containing media, which introduced not only high costs but also significant variability and regulatory hurdles related to sourcing and pathogen safety. The industry-wide shift toward serum-free and, more recently, chemically defined media has been a monumental first step in cost control. These media eliminate the batch-to-batch inconsistency of animal-derived components, reduce contamination risks, and streamline purification processes downstream, thereby delivering savings that ripple through the entire production train.
However, the real frontier in media cost optimization lies in the move toward in-house media preparation. While off-the-shelf powdered or liquid media from commercial vendors offer convenience, they carry substantial price premiums and logistical burdens. An increasing number of biomanufacturers, particularly large-scale producers, are investing in the capability to formulate and blend their own media from bulk raw materials. This approach requires significant upfront investment in quality control infrastructure and expertise to ensure raw material consistency and final media performance. Yet, the long-term payoff can be dramatic, often slashing media costs by 50% or more. Furthermore, this strategy grants process scientists unparalleled flexibility to tweak and optimize formulations in real-time for specific cell lines or process conditions, turning media from a fixed cost into a tunable performance lever.
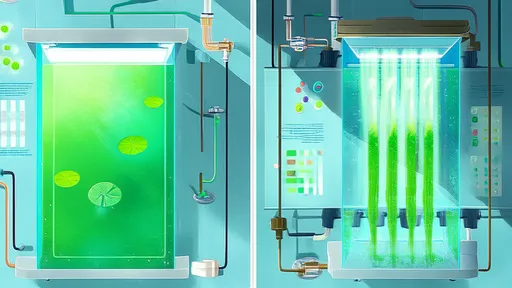
Parallel to the revolution in media is the relentless innovation in bioreactor design and operation. The traditional workhorse, the stainless-steel stirred-tank reactor, involves high capital expenditure (CAPEX) and considerable operational costs for cleaning, sterilization, and maintenance. The advent of single-use bioreactors (SUBs) has been a game-changer, particularly for multiproduct facilities and clinical-scale manufacturing. SUBs eliminate the need for costly clean-in-place (CIP) and steam-in-place (SIP) systems, drastically reduce water and utility consumption, and allow for much faster turnaround between batches. The cost savings here are not just in direct operational expenditure (OPEX) but also in the increased facility utilization and reduced cross-contamination risks, which enhance overall throughput.
Yet, for very large-scale commercial production, the cost of single-use components can become prohibitive. This has spurred a hybrid approach and innovations in reusable systems. New generations of stainless-steel systems are being designed with greater efficiency, incorporating advanced sensors for real-time monitoring and control. The implementation of advanced process control strategies, often powered by machine learning algorithms, represents a massive opportunity for cost reduction in any bioreactor system. By moving beyond traditional set-point control to dynamic, predictive control of parameters like pH, dissolved oxygen, and nutrient feeding, manufacturers can push cells to their maximum productivity, shorten cultivation times, improve batch consistency, and reduce the consumption of expensive media components. This data-driven intensification squeezes more product out of every liter of culture and every hour of reactor time.
The synergy between media and bioreactor optimization is perhaps most evident in the field of process intensification, particularly in fed-batch and perfusion cultures. Fed-batch processes, which involve the controlled addition of nutrients over time, allow for much higher cell densities and product titers than simple batch cultures. This directly reduces the cost per gram of product by maximizing the output from a single bioreactor run. The development of highly concentrated nutrient feeds is key here, enabling high levels of supplementation without excessively diluting the culture or altering its osmotic balance. The choice of feed strategy—whether based on predetermined schedules, metabolic consumption rates, or real-time feedback control—is a critical cost and performance decision.
Perfusion culture takes intensification a step further by continuously feeding fresh media and removing spent media and products, maintaining cells in a optimal productive state for weeks. While perfusion systems are more complex, requiring cell retention devices and continuous processing equipment, they can achieve cell densities an order of magnitude higher than fed-batch. This can dramatically reduce the footprint of production; a 500-liter perfusion reactor can potentially match the output of a much larger traditional batch system. The cost savings in facility size, utility consumption, and capital equipment are enormous. However, this comes with the caveat of significantly higher media consumption. Therefore, the economic viability of perfusion is critically dependent on using a cost-optimized, often proprietary, media formulation. The high media cost can be justified only if the corresponding increase in volumetric productivity is substantial enough.
Looking forward, the next wave of cost reduction will likely be driven by further integration and data analytics. The concept of the digital twin—a virtual, dynamic model of the bioreactor process—is gaining traction. By simulating thousands of process scenarios, scientists can identify the most efficient and cost-effective strategies for media use and bioreactor control before ever running a single experiment. This in silico approach de-risks scale-up and accelerates optimization, saving both time and valuable resources. Additionally, the exploration of alternative, lower-cost raw materials for media, such as plant-derived hydrolysates or sustainably sourced ingredients, continues. The ultimate goal is a fully optimized, agile, and transparent supply chain that minimizes cost at every node without sacrificing quality.
In conclusion, the journey to reduce cell culture costs is a continuous cycle of innovation, integration, and intensification. It is no longer about seeking a single silver bullet but about orchestrating a symphony of improvements across media science, engineering design, and process control. The manufacturers who will lead the next decade are those viewing cost not as a line item to be minimized, but as an outcome of a elegantly designed and deeply understood process. The relentless pressure to make biologics more affordable globally ensures that the pursuit of efficiency in media and bioreactors will remain a central, and thrilling, discipline in biomanufacturing for years to come.

By /Aug 29, 2025

By /Aug 29, 2025
By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025
By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025
By /Aug 29, 2025
By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025
By /Aug 29, 2025

By /Aug 29, 2025
By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025

By /Aug 29, 2025